
A brand of sun screen is recalled due to possible benzene contamination.
The full story below from Ozarks First:
Edgewell Personal Care Company issued a recall Friday for three batches of Banana Boat Hair & Scalp sunscreen sprays after an internal review of product samples found traces of benzene.
Benzene is a human carcinogen that can lead to leukemia, blood cancer in bone marrow, and other blood disorders. People typically are exposed to the chemical in their environment through skin contact, inhalation, or ingestion.
The chemical is not an ingredient in the sunscreen spray, but the FDA said that “unexpected levels of benzene” came out of the spray bottles’ propellant.
As of this report, Edgewater said it has not learned of any illnesses related to the presence of benzene in their products nor does the FDA believe exposure to the benzene in the sprays could be particularly dangerous.
“Daily exposure to benzene in the recalled products would not be expected to cause adverse health consequences according to an independent health assessment using established exposure modeling guidelines,” the FDA advisory said.
However, the FDA said that if you have the affected product, stop using it and throw it out.
The benzene is only linked to the three batches mentioned in the advisory. The FDA provided the following information on the batches affected by the recall:
| UPC | DESCRIPTION | Lot Code | Expiration | Size |
|---|---|---|---|---|
| 0-79656-04041-8 | Banana Boat Hair & Scalp Spray SPF 30 | 20016AF | December 2022 | 6 oz |
| 0-79656-04041-8 | Banana Boat Hair & Scalp Spray SPF 30 | 20084BF | February 2023 | 6 oz |
| 0-79656-04041-8 | Banana Boat Hair & Scalp Spray SPF 30 | 21139AF | April 2024 | 6 oz |
The FDA said Edgewater has told retailers to remove any of the remaining products with the lot codes above from their shelves. If you own a Banana Boat sunscreen spray, you can find the lot code on the bottom of the can.
Those who purchased the affected products can be eligible for reimbursement from Banana Boat, according to the advisory.
If you have had an adverse reaction to the spray, you can report your situation to the FDA MedWatch.





 Local Pantry to Answer Questions about USDA Funding Cuts
Local Pantry to Answer Questions about USDA Funding Cuts
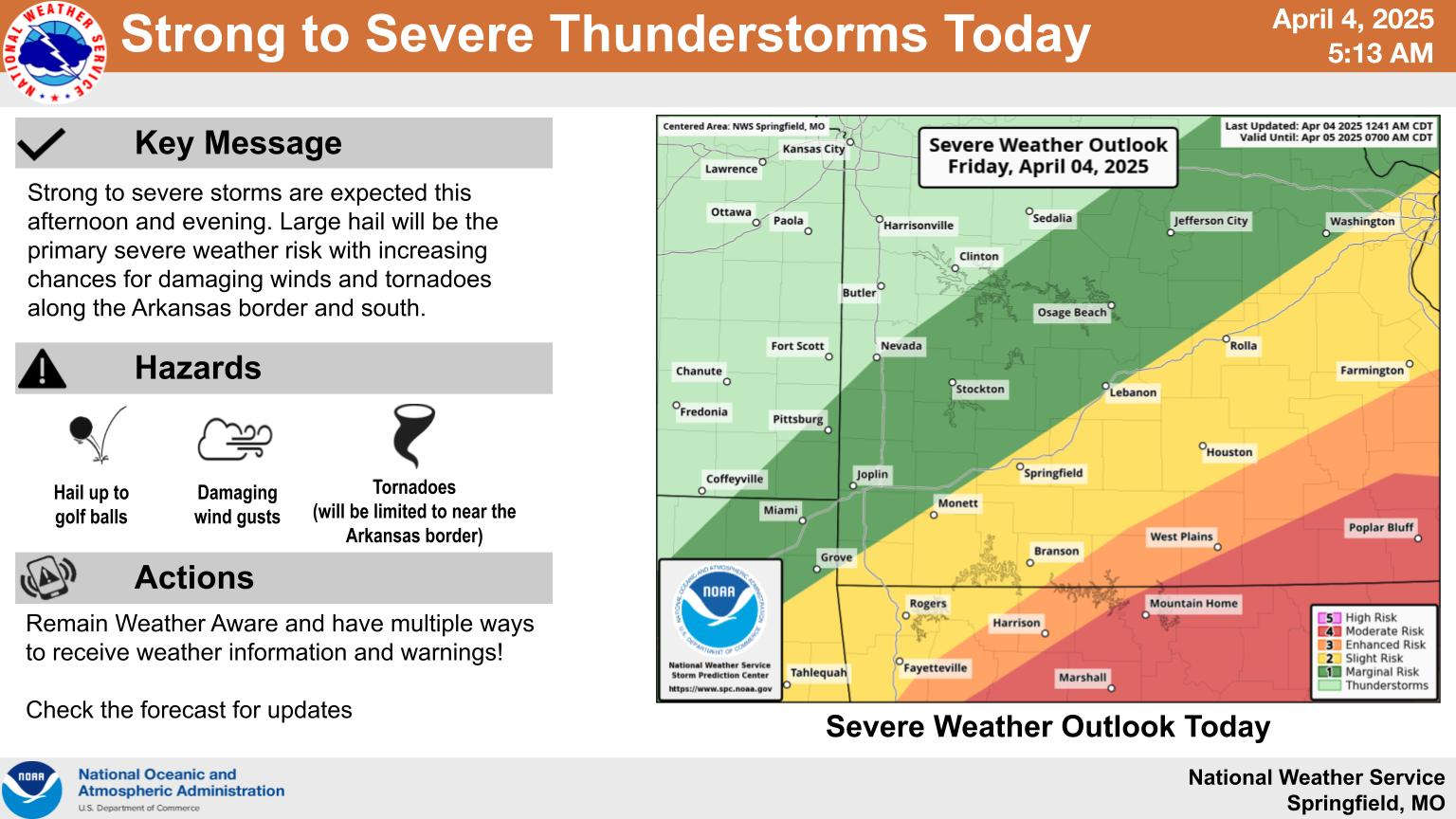 Heavy Rain, Severe Weather Update
Heavy Rain, Severe Weather Update
 Schools Impacted By Storms
Schools Impacted By Storms
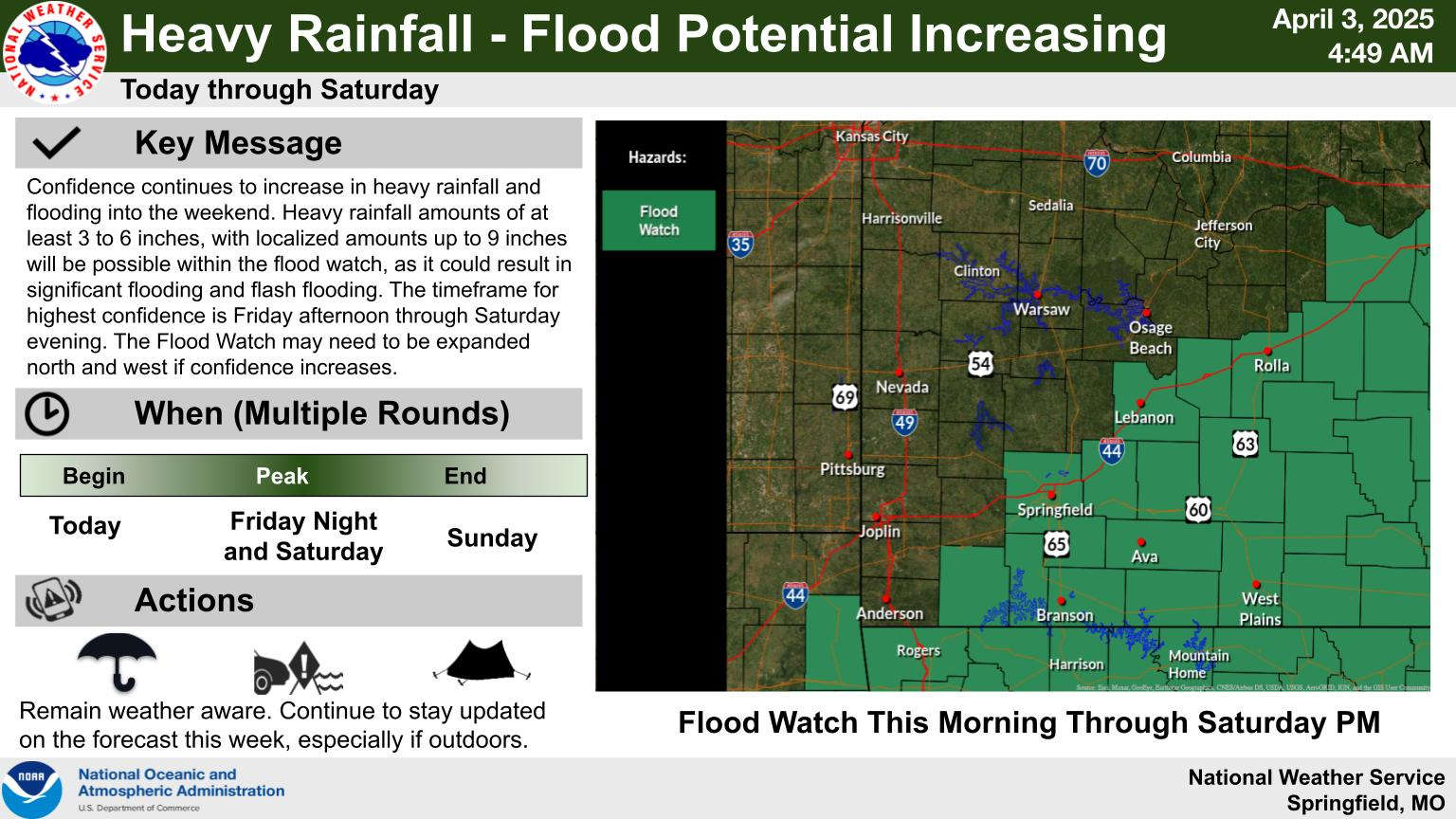 Wednesday Storms, Thursday Rain
Wednesday Storms, Thursday Rain
 Eureka Springs Police Officers Save Lives in Fire
Eureka Springs Police Officers Save Lives in Fire